Initiation Of Antiretroviral Therapy
Antiretroviral drug treatment guidelines have changed over time. Before 1987, no antiretroviral drugs were available and treatment consisted of treating complications from opportunistic infections and malignancies. After antiretroviral medications were introduced, most clinicians agreed that HIV positive patients with low CD4 counts should be treated, but no consensus formed as to whether to treat patients with high CD4 counts.
In April 1995, Merck and the National Institute of Allergy and Infectious Diseases began recruiting patients for a trial examining the effects of a three drug combination of the protease inhibitor indinavir and two nucleoside analogs. illustrating the substantial benefit of combining 2 NRTIs with a new class of anti-retrovirals, protease inhibitors, namely indinavir. Later that year David Ho became an advocate of this “hit hard, hit early” approach with aggressive treatment with multiple antiretrovirals early in the course of the infection. Later reviews in the late 90s and early 2000s noted that this approach of “hit hard, hit early” ran significant risks of increasing side effects and development of multidrug resistance, and this approach was largely abandoned. The only consensus was on treating patients with advanced immunosuppression . Treatment with antiretrovirals was expensive at the time, ranging from $10,000 to $15,000 a year.
Treatment as prevention
New Mechanisms For New Anti
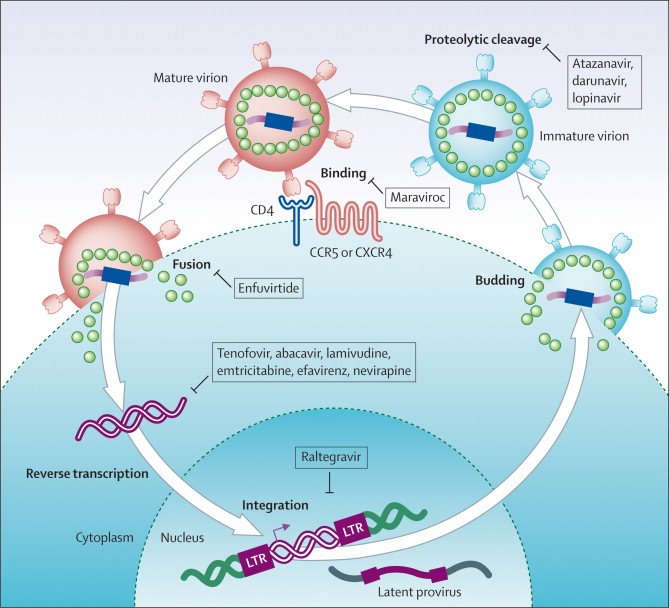
The 3 classes of drugs mentioned above all inhibit HIV replication by mechanisms operating inside the infected cell. The main feature of the new antiretroviral drugs is that they operate outside the cell. These new drugs act by inhibiting the entry of HIV into the target cell, thereby halting the very first step of HIV replication. In addition to their novel mechanism of action, these new drugs also have potential action against drug-resistant HIV strains, cause minimal adverse effects, and may be administered in a simplified dosing regimen .
The process of HIV entry into cells requires 3 steps: attachment of the virus to the target cell membrane, binding of the virus to coreceptors present at the cell surface, and fusion of the virus with the cell membrane to inject its RNA. There are new drug candidates that act at each of these steps .
New drugs under development as of March 2004.
The second step in the process of HIV entry, which results after gp120 binds to the CD4 receptor, is the interaction of gp120 with cellular coreceptors. Indeed, CD4 binding induces conformational changes to expose a region of gp120 that participates in coreceptor binding. There are 2 types of coreceptors, CXCR4 and CCR5 . Both of these molecules are chemokine receptors, which are present in different types of cells, especially in macrophages, monocytes, and T cells. CXCR4 and CCR5 act as coreceptors for HIV-1 in these cells .
The Bottom Line On Covid
The biggest potential risks for interactions with COVID-19 treatments and HIV treatments are those for renal and liver functions. But until the data are in, this remains only a potential concern. Meanwhile, Shapiro says the biggest danger a PLWH will typically face if they get COVID-19 is the danger associated with an unsuppressed HIV viral load.
If a patient has COVID-19 and HIV, they should be given indicated treatments for COVID, and get viral load for HIV suppressed. During COVID or any time, its important to suppress viral load, which can help fight off COVID infection, she said.
Shapiro brought up another complication for PLWH during the COVID crisis, one that might worsen as new cases continue to rise: isolation affecting their access to care. As more people are encouraged to stay home and use telemedicine, its important that theyre able to access .
Similarly, HIV-negative people need resources for testing and case notification, she added. For people who dont know their status, HIV self-testing kits, some of which can be purchased online, could be a helpful alternative to in-person testing during the COVID crisis.
Cachay said its also important for care providers to counsel PLWH about COVID-19 related stress, anxiety, and among some, PTSD-like feelings due to the internalized stigma associated with the AIDS isolation in the 1980s.
You May Like: What Is The Hiv Rate In Atlanta
Patients Cured Of Hiv Infection
The so-called “Berlin patient” has been potentially cured of HIV infection and has been off of treatment since 2006 with no detectable virus. This was achieved through two bone marrow transplants that replaced his immune system with a donor’s that did not have the CCR5 cell surface receptor, which is needed for some variants of HIV to enter a cell. Bone marrow transplants carry their own significant risks including potential death and was only attempted because it was necessary to treat a blood cancer he had. Attempts to replicate this have not been successful and given the risks, expense and rarity of CCR5 negative donors, bone marrow transplant is not seen as a mainstream option. It has inspired research into other methods to try to block CCR5 expression through gene therapy. A procedure zinc-finger nuclease-based gene knockout has been used in a Phase I trial of 12 humans and led to an increase in CD4 count and decrease in their viral load while off antiretroviral treatment. Attempt to reproduce this failed in 2016. Analysis of the failure showed that gene therapy only successfully treats 11-28% of cells, leaving the majority of CD4+ cells capable of being infected. The analysis found that only patients where less than 40% of cells were infected had reduced viral load. The Gene therapy was not effective if the native CD4+ cells remained. This is the main limitation which must be overcome for this treatment to become effective.
Tips For Staying On Your Treatment Plan
Before you start a treatment plan, you should:
- Get your health care provider to write everything down for you: names of the drugs, what they look like, how to take them , and how often to take them. This way, you’ll have something to look at in case you forget what you’re supposed to do.
- With your provider’s help, develop a plan that works for you.
Recommendations For Initiation Of Antiretroviral Therapy
The Adult and Adolescent ARV Guidelines recommend initiation of antiretroviral therapy for all persons with HIV to reduce morbidity and mortality associated with HIV infection and to prevent HIV transmission to others . In addition, antiretroviral therapy should be started immediately, or as soon as possible, after the HIV diagnosis.
Blocking The Virus From Entering The Cell
The first steps of the HIV infection cycle, where HIV attaches to and enters the human cell, can be blocked with compounds known as;entry;or;fusion inhibitors.;
- CCR5 blockers, such as , as well as CXCR4 blockers, are examples of these ARV drugs. They work by blocking cell receptors, called CCR5 and CXCR4, respectively,;and prevent HIV from attaching to the host cell, interrupting the HIV life cycle in its earliest stages.;
- gp120 inhibitors, such as DS003, bind to the gp120 proteins HIV needs to attach to healthy cells. Like CCR5 and CXCR4 blockers, gp120 inhibitors prevent the virus from attaching to and entering healthy cells.
- gp41 inhibitors can also interfere with these first steps by blocking the viral gp41 protein and therefore the ability of HIV to fuse with human immune cells.;
Mechanisms Of Hiv Antiretroviral Drug Action And Drug Resistance
Nucleoside reverse transcriptase inhibitors
Mechanism of resistanceThere are two ways in which NRTI resistance can be achieved:
1. Discrimination pathwayThese;resistance mutations;are;amino acid;changes in the;primary structure;of ;reverse transcriptase;that increase the selective ability of the enzyme to incorporate the natural ;nucleotide;over the NRTI-triphosphate;.
There are two ways in which discrimination can be achieved:
How Do People Develop Drug Resistance
Acquired HIV drug resistance can happen when a person has HIV that is replicating , but is also taking a particular antiretroviral medication. HIV can mutate around that medication. This will result in HIV being resistant to the medications and those medications now being ineffective. In most studies, more than 70 80% of people with virological failure develop acquired HIV drug resistance.
Although acquired drug resistance can occur if a person does not maintain good adherence to their HIV medications, sometimes the drugs themselves or a combination of how a persons body reacts to the drug can also cause drug resistance. Even if you maintain perfect adherence, you may experience poor absorption. This means that the drugs dont get absorbed by your body easily and arent preventing HIV from replicating, which can cause drug resistance.
Sometimes, drugs with less than optimal pharmacokinetics can cause drug resistance. This means that the drugs arent effective because they arent moving efficiently and sufficiently within your body.
Pretreatment HIV drug resistance can occur before treatment is even started. This may occur if a person is exposed to HIV medications when they become infected with HIV. For instance, if a women is taking drugs for prevention of mother-to-child HIV transmission or if a person is taking pre-exposure prophylaxis , and then that person becomes infected with HIV, it is theoretically possible for that person to develop drug-resistance.
When Should You Start Hiv Treatment
Treatment guidelines from the U.S. Department of Health and Human Services recommend that a person living with HIV begin ART as soon as possible after diagnosis. Starting ART slows the progression of HIV and can keep you healthy for many years.
If you delay treatment, the virus will continue to harm your immune system and put you at higher risk for developing opportunistic infections that can be life threatening.
Antiretroviral Drug Side Effects And Management
HIV drugs have improved over the years, and serious side effects are less likely than they used to be. However, HIV drugs can still cause side effects. Some are mild, while others are more severe or even life-threatening. A side effect can also get worse the longer a drug is taken.
Its possible for other medications to interact with HIV drugs, causing side effects. Other health conditions can also make the side effects from HIV drugs worse. For these reasons, when starting any new drug, people with HIV should tell their healthcare provider and pharmacist about all the other medications, supplements, or herbs theyre taking.
In addition, if any new or unusual side effects occur, people with HIV should call their healthcare provider. They should do this even if theyve been on the medication for a long time. It can take months or years to start reacting to a drug.
For serious side effects, a healthcare provider might make sure that its the medication and not another factor thats causing the symptoms. If the drug is to blame, they might switch treatment to another antiretroviral drug. However, switching treatments isnt easy. They need to be sure that the new treatment will still work and that it wont cause even more severe side effects.
Here are some of the more common side effects from antiretroviral drugs and tips for managing them.
Examples of drugs that may cause it:
- abacavir
What might help:
Takeaways For Avoiding Drug Resistance
Drug Interactions For Azt
The following drugs interact or have the potential to interact with AZT. These lists are not exhaustive.
AZT should never be combined with d4T , as these drugs interfere with each other.
The following drugs can affect the bone marrow, decreasing the production of white and/or red blood cells. Using AZT with these or other drugs that affect the bone marrow can increase the risk of infections and/or anemia:
- dapsone
- ribavirin
- valganciclovir
- valproic acid
AZT should be used cautiously with these drugs, or not at all.
For some people, but not all, methadone increases the blood level of AZT. Aspirin, codeine, morphine and a number of other drugs can also affect the metabolism of AZT, so use of these drugs should be discussed with your doctor.
The antibiotic clarithromycin can reduce the absorption of AZT. This can be avoided by taking the two medications at least two hours apart.
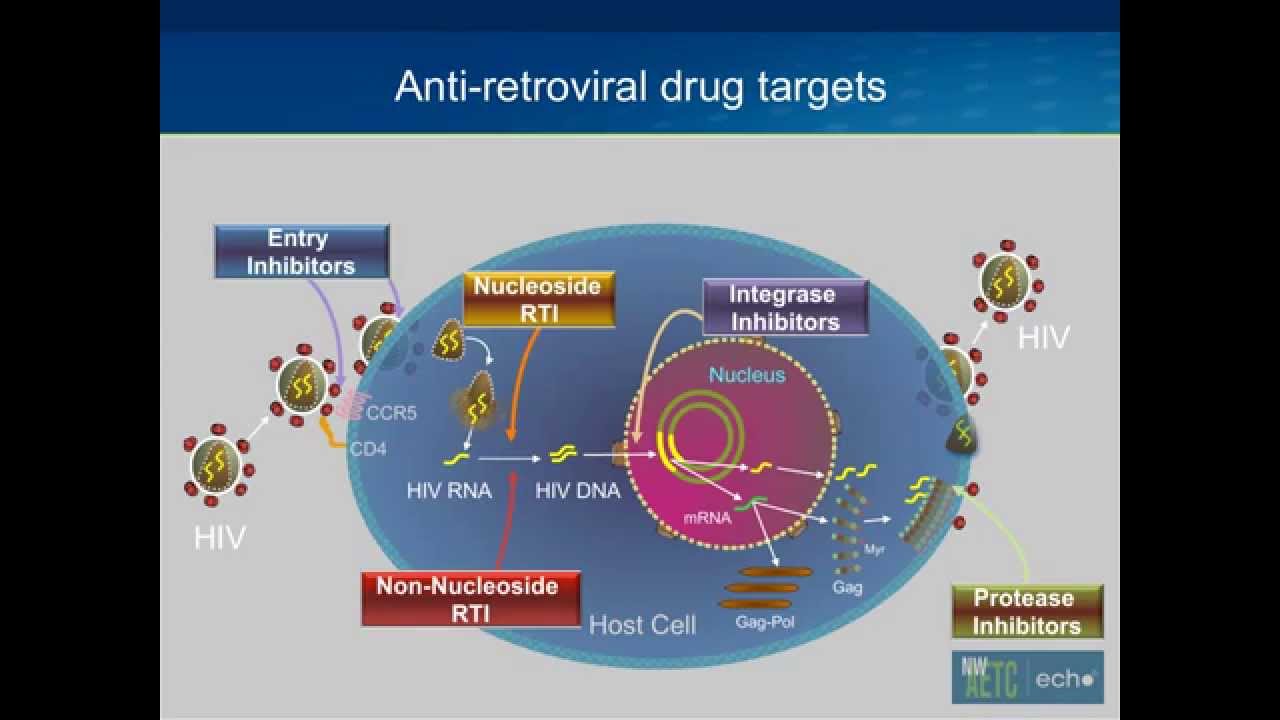
Reverse Transcription And Reverse Transcriptase Inhibitors
HIV Reverse Transcriptase
The HIV reverse transcriptase enzyme is the enzyme involved in the critical HIV reverse transcription process. The HIV reverse transcriptase enzyme is a heterodimer consisting of the p66 and p51 subunits; the p66 and p51 subunit are 560 and 440 amino acids in length respectively, with the 440 amino acids of p51 overlapping with the first 440 amino acids of the p66 subunit . The p66 subunit, which primarily has a catalytic role, consists of the polymerase and RNase H domains. Conceptually, the polymerase domain is structurally analogous to a human right hand, with specific regions corresponding to fingers, palm, and thumb. The p51 subunit functions have a structural role, and it is very closely related to, but not identical with, the polymerase domain of the p66 subunit. Each HIV-1 virion contains approximately 50 reverse transcriptase enzymes.
Reverse Transcription
Reverse Transcriptase Inhibitors
The HIV reverse transcriptase inhibitors include two classes of antiretroviral medications: the nucleoside reverse transcriptase inhibitors and the non-nucleoside reverse transcriptase inhibitors . Mechanistically, the fundamental difference between these classes is that the NRTIs act as host nucleotide decoys and cause termination of the elongating HIV DNA chain whereas the NNRTIs bind directly to the HIV reverse transcriptase enzyme and inhibit the function of the enzyme.
How Do You Deal With Side Effects
Some side effects can be hard to deal with. One way to cope with them is to know what to watch out for and have a plan to deal with problems that come up.
That’s why you need to talk to your provider about the risk of side effects from different drugs, before you start therapy.
At the beginning of any treatment, you go through a period of adjustment–a time when your body has to get used to the new drugs you’re taking. Sometimes you’ll have headaches, an upset stomach, fatigue, or aches and pains. These side effects may go away after a few days or a few weeks.
If you notice any unusual or severe reactions after starting or changing a drug, report the side effects to your provider immediately.
More information is available in the Side Effects Guide.
How To Use Abacavir
Read the Medication Guide and Warning Card provided by your pharmacist before you start taking abacavir and each time you get a refill. Carry the Warning Card with you at all times. If you have any questions, ask your doctor or pharmacist.
Take this medication by mouth, usually 1-2 times daily with or without food or as directed by your doctor. If you are using the liquid form of this medication, carefully measure the dose using a special measuring device/spoon. Do not use a household spoon because you may not get the correct dose.
The dosage is based on your medical condition and response to treatment. In children, the dosage is also based on weight.
If you stop using abacavir even for a short time and then restart the drug, you have an increased chance of developing a very serious allergic reaction. Refill your medication before you run out. Do not stop treatment unless directed by your doctor. Before restarting abacavir, consult your doctor or pharmacist, and be sure you have easy access to medical care.
It is very important to continue taking this medication exactly as prescribed by your doctor. Do not skip any doses. Do not increase your dose, take this drug more often than prescribed, or stop taking it even for a short time unless directed to do so by your doctor. Skipping or changing your dose without approval from your doctor may cause the amount of virus to increase, make the infection more difficult to treat , or worsen side effects.
Articles On Hiv Treatments
- rilpivirine/ tenofovir disoproxil fumarate/emtricitabine, or RPV/TDF/FTC
- rilpivirine/tenofovir alafenamide/emtricitabine, or RPV/TAF/FTC
- tenofovir alafenamide/emtricitabine, or TAF/FTC
- tenofovir disoproxil fumarate, or TDF
- tenofovir disoproxil fumarate/emtricitabine, or TDF/FTC
WebMD does not provide medical advice, diagnosis or treatment.