When Should You Start Hiv Treatment
If you have HIV, its important to start treatment with HIV medicine as soon as possible after diagnosis,regardless of how long youve had the virus or how healthy you are. HIV medicine slows the progression of HIV and can keep you healthy for many years.
It is especially important for people with HIV who have early HIV infection or an AIDS-defining condition to start HIV medicines right away.
People with HIV who become pregnant and are not already taking HIV medicines should also start taking HIV medicines as soon as possible to protect their health and to prevent transmitting HIV to their babies.
If you have been diagnosed with HIV and are not currently taking HIV medicines, talk to a health care provider about the benefits of getting on treatment.
Symtuza Approved: A 4
In 2018, the FDA approved Janssens four-drug regimen Symtuza for HIV treatment. Individually, the drugs in Symtuza include a protease inhibitor, an antiviral booster, and 2 nucleoside reverse transcriptase inhibitors.
Symtuza is a darunavir-based, single-tablet regimen given once daily for the treatment of HIV-1 in treatment-naïve and certain virologically suppressed patients. Darunavir provides a high barrier to drug resistance.
Symtuza is approved to be used in adults and pediatric patients weighing at least 40 kg :
- who have no prior antiretroviral treatment history or
- who are virologically suppressed on a stable antiretroviral regimen for at least 6 months and have no known resistance associated with darunavir or tenofovir.
- The Symtuza dose is one tablet once daily with food in patients weighing at least 40 kg. For patients who are unable to swallow the whole tablet, Symtuza may be split with a tablet-cutter and taken immediately after splitting.
In two Phase 3 studies, Symtuza was compared to control regimens in those with no prior antiretroviral therapy history and in virologically suppressed adults . Symtuza was found effective and well-tolerated, with up to 95% of patients, achieving or maintaining virologic suppression in both studies.
Side effects included:
- stomach side effects, such as: diarrhea, intestinal gas, nausea, stomach pain
- rash
- headache.
Satisfying Patient Needs: The Evolving Challenge
Dr Kimberly Smith, Head of Research and Development at ViiV Healthcare, explained that as treatments have developed, so have patients expectations and needs: the bar is set higher and higher because the medicines are increasingly effective and well tolerated, especially with the introduction of two-drug regimens. Patients, she said, are still hoping that a cure will be developed but in the meantime their needs have evolved. According to Smith, where single-pill regimens were once a major unmet need, with multiple single-tablet regimens now on the market, the needs of patients have changed: people now want to be able to not have to think about living with HIV every day.
This, she said, is the premise upon which long-acting therapies have entered the market: People want to have the privacy that comes with not having medicines in their home or not needing to travel with them. What long-acting therapy does is to take away that daily reminder of their disease. The first long-acting cART regimen for HIV Janssens Rekambys and ViiVs Vocabria injection was approved for once monthly dosing in the US in January 2021 and EU in December 2020, the EU has also approved the two-month dosing regimen.6
though it is unlikely a cure for HIV will be developed in the next ten years, the industry should be dosing patients with less frequency
Recommended Reading: How Long Can You Have Hiv Without Testing Positive
Summary Over The Last Year
Main news over the last year included:
- Approval of cabotegravir/rilpivirine LA in EU and US in January 2021, although the NHS process for access is still ongoing.
- Approval of fostemsavir for HIV MDR in EU and US in January 2021 and July 2020 respectively.
- Phase 2/3 results using islatravir , including in dual ART with doravirine. Pharmacokinetic data supporting a single monthly pill for use as PrEP.
- Once-weekly NNRTI MK-8507 is now in phase 2 studies in combination with weekly islatravir. . .
- Early results were presented for oral and subcutaneous formulations of maturation inhibitor from ViiV and both treatment-naïve and MDR treatment.
- Gilead and Merck/MSD announced a new partnership for developing long-acting formulations.
- Long-acting capsid inhibitor lenacapravir has clinical results in naive and drug resistance, plus animal data for PrEP. Lenacapavir is given every 6 months.
- Limited phase 3 results on albuvirtide, given as a weekly injection.
- bNABs: developments include results from AMP studies using VRC01 as prevention. Numerous other studies on bNAbs used in combinations in prevention and cure-related studies.
- Combinectin, MK-8504 and MK-8583 were discontinued.
Maturation Inhibitors: Gsk3640254 And Gsk3739937

Maturation inhibitors work at the late stage of the viral lifecycle by producing non-infectious undeveloped HIV. As with other new classes, maturation inhibitors will not be cross-resistant to other types of HIV drugs.
GSK have two second-generation compounds in this class.
CROI 2021 included results on GSK3640254 from a phase 2a two-stage dose-finding study in 34 treatment-naive participants . Oral dosing was once-daily and given with a moderate fat meal.
In stage 1, participants were randomised to either 10 mg of 200 mg for ten days. In part two, doses were 40 mg, 80 mg or 140 mg for seven days. Follow-up in stage 1 continued without treatment from days 11 to 17, with ART started on day 18. In stage 2, ART was started on day 8.
Mean age was 31, 94% were men and mean baseline viral load range from about 15,000 to 65,000.
Changes in viral load were roughly proportional to dose, with mean changes in plasma viral load ranging from 2.0 to 0.2 log copies/mL. The greatest mean reductions of 2.0 and 1.5 log were greatest in the 200 mg and 140 mg groups, respectively.
However, 4/6 participants in the 200 mg arm in stage 1 developed drug resistance at day 11 with A364A/V partial mixed variant which by day 21 had developed into the full mutation in 1/4 with 132-fold phonotypic resistance. No resistance was seen in the 10 mg arm but the results prompted the reduction to 7 days monotherapy in stage 2 .
Both compounds are being developed by GSK/ViiV.
Recommended Reading: Can Hiv Be Transmitted If On Medication
Hiv Vaccine And Cure Research In 202: More Hope Than Reality
Largely because HIV is a very wily and slippery virus and because we still have nearly no clue about why a small handful of people seem to be able to control HIV with their own immune systems, theres nothing very ripe on the horizon when it comes to either HIV vaccines or cures.
Vaccine-wise, 2021 was yet another groaner, as the long-awaited results of the Imbokodo trial proved disappointing. On the cure side, although researchers discovered the second woman ever whose own immune system seems to have eradicated HIV completely, theres currently nothing weve extracted from such cases to chart a path toward an intervention that would help the rest of us get there.
So whats the take-home as we go into 2022? Says Harrington: We continue to see exciting developments in prevention and treatment, but at the same time, were failing grievously to get treatment and PrEP to the people who need it, both domestically and globally.
Does this mean we should consider halting new developments while we try to maximize use of the very good tools we already have? No, says Harrington: A breakthrough technology can potentially drive a huge new amount of demand.
Tim Murphy
Vocabria: An Oral Lead
In Jan. 2021 the FDA approved Vocabria 30 mg tablets, used in combination with Edurant 25 mg tablets for the short-term oral treatment of HIV-1 infection in adults to replace their current HIV-1 medicines. It is not known if Vocabria is safe and effective in children.
Vocabria + Edurant is indicated for patients who are virologically suppressed on a stable antiretroviral regimen with no history of treatment failure and with no known or suspected resistance to either cabotegravir or rilpivirine.
- Patients may receive Vocabria + Edurant for one month before they receive Cabenuva extended-release intramuscular suspension for the first time. Vocabia as a single agent may also be used to assess tolerability one month before starting Apretude injections for PrEP. This will allow the healthcare provider to assess how well cabotegravir or rilpivirine is tolerated.
- Vocabria can also be used as oral therapy for people who will miss planned injections with Cabenuva.
- The most common side effects with Vocabria include: headache, nausea, abnormal dreams, anxiety and sleep disorders.
Read Also: How To Stop Spread Of Hiv
Other Hiv Treatment Possibilities On The Horizon
Many other candidates that work in different ways are in very early stages, some of them being designed specifically for low-income countries. However, most drugs dont make it past early trials, although occasionally some of them, such as MK-8507, are revived as scientists test them out alongside another investigational agent.
The big unknown, says Harrington, concerns the emerging field of long-acting meds. Getting HIV treatment down to once every six months would be a game-changer, he says, but those pills would probably be $25,000 to $40,000 each. Are they going to bring your meds to you in a Brinks truck? This could be transformative in the next decade, but the jurys still out on how accessible theyll be.
Identifying The Cause Of Aids
In 1983, scientists determined that a then-novel retrovirus is the cause of AIDS . HIV is a retrovirus that attacks CD4+ T cells, which play a central role in helping the immune system to recognize pathogens and mount an appropriate defense. HIV replicates within these cells with the aid of three virally encoded enzymes: reverse transcriptase, integrase, and protease . Reverse transcriptase is used to produce DNA from the virus RNA genome, which is integrated into the host cells DNA with the help of the integrase enzyme. Protease cleaves protein precursors to build new, complete viral particles, which assemble at the cell surface and are released to infect other CD4+ T cells in the body. As the population of T cells is depleted, the immune system slowly loses its ability to effectively protect itself. If left untreated, HIV infection will usually progress to AIDS. While HIV/AIDS is not itself fatal, the resulting susceptibility to opportunistic infection and cancer is deadly .
Recommended Reading: How Soon Do Hiv Symptoms Appear
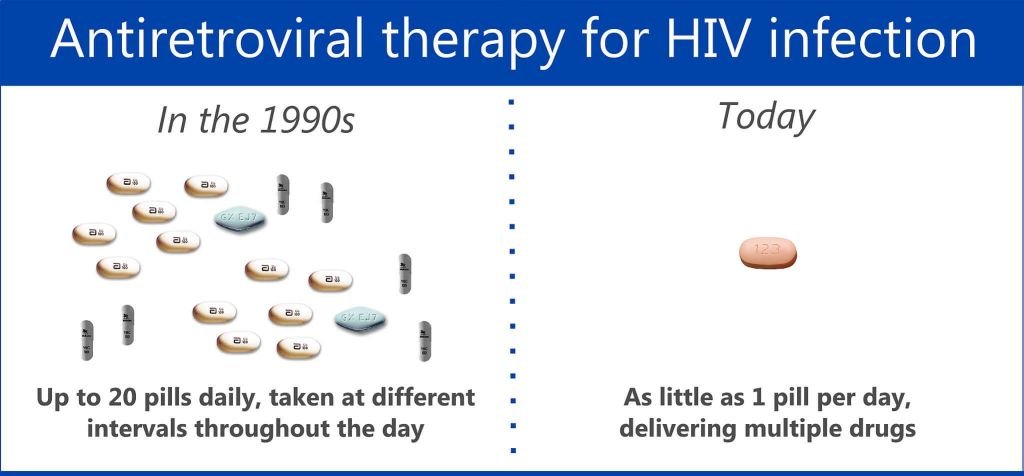
Hiv Advances: One Convienent Pill
HIV treatments involve several antiretroviral therapy medications, often combined into one convenient pill. Combination treatments:
- make it easier to stay on course
- fight drug resistance
- are often given once-a-day.
No more taking handfuls of pills every few hours.
Experts recommend starting HIV treatment as soon as diagnosed, regardless of the T-cell count .
Plus, many of the latest combinations are recommended to be used in “treatment-naive” patients — that is, patients who have never received any HIV medication. HIV drug-resistance testing is recommended in all patients.
Milestones And Recent Advances In Hiv Research
Acquired immunodeficiency syndrome refers to a number of infections and illnesses that occur when the immune system has been compromised by the human immunodeficiency virus . Since the first known case in 1959, over 79.3 million people have been infected by HIV worldwide, and 36.3 million people have died from an AIDS-related illness .
HIV can be transmitted through the exchange of certain bodily fluids including semen, blood, breast milk, and vaginal fluids . Common sources of HIV infection include unprotected sex and the sharing of needles. Importantly, HIV cannot be transmitted through saliva, tears, sneezing, and ordinary physical contact such as hugging or kissing.
Despite the many advances made in AIDS research and therapies since the 1980s, stigma and misconceptions surrounding AIDS persist to this day. In honor of World AIDS Day, InsideScientific has set out to provide information on the historical significance of HIV and AIDS, highlight recent advances in anti-HIV therapy, and describe the impact that COVID-19 has had on HIV testing, diagnosis and treatment around the world.
World AIDS Day Vector Artwork by Open Art Source: shutterstock
Read Also: What To Do If I Have Hiv
The Evolution Of Hiv Treatments
Thirty years ago, healthcare professionals didnt have encouraging news to offer people whod received a positive diagnosis of HIV. Today, its a manageable health condition.
Theres no HIV or AIDS cure yet. However, remarkable advancements in treatments and clinical understanding of how HIV progresses are allowing people with HIV to live longer, fuller lives.
Lets look at where HIV treatment is today, the effects new therapies are having, and where treatment may be headed in the future.
Introduction: Pipeline 2021 After Covid
This report reviews developments over the last 14 months since the last edition produced for CROI 2020.
It is also updated to include important results presented at the IAS 2021 virtual conference.
The HIV pipeline this year is exciting and significant.
The three main research-based drug companies Gilead Sciences, Merck/MSD and ViiV Healthcare are all focused on simplified ART. All have current or planned dual regimens supported by the greater potency of recent drugs. These companies also have long-acting compounds in new drug classes. If effective, within a few years, we could have many alternatives to daily oral ART.
This is also a unique time because many of the pipeline compounds have the potential to be used for both treatment and prevention. As well as this, for the first time in many years, much of the current pipeline comes from new drug classes that could be used for people who are treatment-naive, treatment-experienced, and with multidrug resistant HIV.
The same companies developing new drugs for treatment and prevention are also investing in strategies to cure HIV.
Now, the research challenge is not only to produce more effective and tolerable treatments and this bar is already set high but to make sure that these are affordable. And that the participants in registrational studies reflect the demographics of the global HIV population.
Nevertheless, this is an exciting time. The 2021 pipeline report includes updates on the following drugs:
Recommended Reading: Can Hiv Have No Symptoms
Developments To Watch For In Hiv/aids Treatment And Prevention In 2021
Injectable treatments may start to replace daily pills.
2021 stands to be pivotal year for HIV/AIDS prevention and treatment. Many researchers and advocacy groups are heartened by President-elect Joe Bidens selection of Rochelle Walensky, M.D., M.P.H., to be the CDC director of the new administration. Walensky, chief of infectious diseases at Massachusetts General Hospital and a professor at Harvard Medical School, is a well-known HIV/AIDS researcher. The CDC director does not require Senate confirmation so theres no reason to believe that Walensky wont heading up the federal governments prime infectious disease agency.
Walensky will be taking the reins at the CDC at a crucial time in the course of the HIV/AIDS epidemic. Many people infected with HIV dont have a diagnosis, so diagnosed cases don’t tell the full story by any means, Even so, it’s notable that the number of diagnosed cases has been declining. In 2018, the latest year for which a tally is available, 37,968 new cases were diagnosed, which is 2,868 fewer cases than the 40,836 diagnosed in 2014. In 2018, 5,425 Americans died from the AIDS.
Two years ago, the Trump administration promulgated a 10-year plan to end to end the HIV epidemic. The plan largely hinges on the availability and uptake of medications to both prevent and treat HIV, so medical innovation and ways to disseminate such innovation are crucial.
Update From Ias 2021 Conference
Although much of this report covers advances over the last year, the IAS 2021 conference included new data on many pipeline compounds.
This included studies on long-acting cabotegravir/rilpivirine, fostemsavir, lenacapavir , islatravir and albuvirtide.
- Several implementation studies showed that practical aspects of prescribing long-acting injectable ART can be overcome.
- Potential baseline resistance to cabotegravir/rilpivirine LA in a large French database reporting that 7% of > 4000 treatment naive samples showed resistance to rilpivirine.
- Two posters on fostemsavir: on side effects out to 96 weeks and drugs in the background regimen of the BRIGHTE study.
- Efficacy and safety data on paediatric dolutegravir from the large international ODYSSEY study in treatment-naive and -experienced children.
- 96-week phase 2 safety data on islatravir/doravirine dual ART, including bone and kidney results, and use in renal disease.
- Islatravir PrEP studies included PK data easily supporting once-monthly oral pill and plans to include islatravir in a vaginal ring .
- Two PK posters on GS-8507 showing no drug interactions with either islatravir or oral contraceptives. Note: development of compound was stopped in November 2021).
- bNAbs: use in cure related research, susceptibility in the AMP studies,
- Albuvirtide phase 3 results: dual ART with lopinavir
Recommended Reading: Which Of The Following Fluids Cannot Transmit Hiv
A Awaiting A Decision From The Fda On Long
So far, treatment for HIV involves taking pills that need to be taken daily. That may change this year with the advent of treatments that can be injected and dont need to be taken daily. The implications of long-acting injectable treatment are great, as they will likely improve adherence to treatment and eliminate the daily reminder of treatment.
The FDA is expected to issue its decision on ViiV Healthcares injectable Cabenuvaa combination of cabotegravir and rilpivirinesometime in the next few months.
ViiV Healthcare, a joint venture by GlaxoSmithKline and Pfizer, is marketing Cabenuva.
In phase 2 and 3 clinical trials, the treatment combination, given as two injections one of cabotegravir and the other, rilpivirine once a month by a healthcare provider, was shown to be equally as effective as traditional oral options. While it did cause injection-site reactions, patients still preferred Cabenuva over the alternative daily oral option.